What Size Tank Would Be Needed To Contain This Same Amount Of Helium At Atmospheric Pressure (1 Atm)
Chapter 9. Gases
9.1 Gas Pressure
Learning Objectives
By the stop of this section, you lot volition be able to:
- Define the belongings of pressure
- Define and catechumen amongst the units of force per unit area measurements
- Describe the operation of mutual tools for measuring gas force per unit area
- Summate force per unit area from manometer data
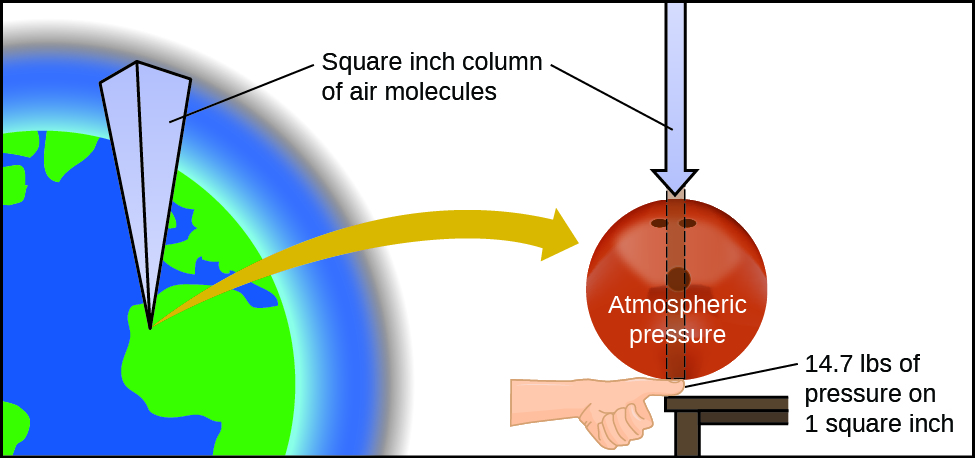
The globe's temper exerts a pressure, every bit does any other gas. Although we practice non unremarkably observe atmospheric pressure level, we are sensitive to pressure changes—for example, when your ears "pop" during take-off and landing while flight, or when you dive underwater. Gas pressure level is caused past the force exerted by gas molecules colliding with the surfaces of objects (Figure i). Although the force of each collision is very small, any surface of observable area experiences a large number of collisions in a short time, which tin consequence in a high pressure. In fact, normal air pressure is strong plenty to crush a metal container when not counterbalanced by equal pressure from inside the container.

A dramatic illustration of atmospheric pressure is provided in this brief video, which shows a railway tanker auto imploding when its internal pressure level is decreased.
A smaller scale demonstration of this phenomenon is briefly explained.
Atmospheric force per unit area is caused by the weight of the column of air molecules in the atmosphere above an object, such as the tanker car. At sea level, this pressure is roughly the same equally that exerted by a full-grown African elephant standing on a doormat, or a typical bowling brawl resting on your thumbnail. These may seem similar huge amounts, and they are, but life on earth has evolved under such atmospheric pressure. If you actually perch a bowling brawl on your thumbnail, the pressure experienced is twice the usual pressure, and the sensation is unpleasant.
In full general, force per unit area is divers as the force exerted on a given area: [latex]P = \frac{F}{A}[/latex]. Note that force per unit area is directly proportional to force and inversely proportional to area. Thus, pressure can be increased either by increasing the amount of force or by decreasing the area over which it is applied; pressure can be decreased by decreasing the force or increasing the area.
Permit'due south apply this concept to decide which would be more probable to fall through thin ice in Effigy ii—the elephant or the figure skater? A large African elephant can weigh 7 tons, supported on four feet, each with a bore of about 1.v ft (footprint area of 250 in2), and so the pressure exerted past each pes is about 14 lb/in2:
[latex]\text{pressure per elephant human foot} = 14,000 \frac{\text{lb}}{\text{elephant}} \times \frac{1 \;\text{elephant}}{four \;\text{feet}} \times \frac{1 \;\text{foot}}{250 \;\text{in}^2} = fourteen \;\text{lb/in}^two[/latex]
The figure skater weighs near 120 lbs, supported on two skate blades, each with an area of nearly 2 in2, so the pressure exerted by each blade is about 30 lb/intwo:
[latex]\text{pressure per skate blade} = 120 \frac{\text{lb}}{\text{skater}} \times \frac{one \;\text{skater}}{2 \;\text{blades}} \times \frac{1 \;\text{bract}}{2 \;\text{in}^2} = 30 \;\text{lb/in}^2[/latex]
Fifty-fifty though the elephant is more than i hundred-times heavier than the skater, it exerts less than one-one-half of the force per unit area and would therefore be less likely to fall though thin water ice. On the other hand, if the skater removes her skates and stands with bare anxiety (or regular footwear) on the ice, the larger expanse over which her weight is applied greatly reduces the pressure exerted:
[latex]\text{pressure per human foot} = 120 \frac{\text{lb}}{\text{skater}} \times \frac{1 \;\text{skater}}{2 \;\text{feet}} \times \frac{1 \;\text{foot}}{30 \;\text{in}^2} = 2 \;\text{lb/in}^2[/latex]

The SI unit of pressure is the pascal (Pa), with i Pa = i N/m2, where North is the newton, a unit of force defined as 1 kg thou/southtwo. 1 pascal is a minor pressure level; in many cases, it is more convenient to utilize units of kilopascal (one kPa = 1000 Pa) or bar (1 bar = 100,000 Pa). In the United states, pressure is ofttimes measured in pounds of force on an area of one square inch—pounds per square inch (psi)—for instance, in car tires. Pressure can also be measured using the unit atmosphere (atm), which originally represented the average sea level air pressure at the approximate latitude of Paris (45°). Tabular array 1 provides some information on these and a few other mutual units for pressure measurements
| Unit of measurement Proper name and Abbreviation | Definition or Relation to Other Unit |
|---|---|
| pascal (Pa) | 1 Pa = 1 Due north/m2 recommended IUPAC unit of measurement |
| kilopascal (kPa) | 1 kPa = yard Pa |
| pounds per square inch (psi) | air pressure level at ocean level is ~fourteen.7 psi |
| temper (atm) | 1 atm = 101,325 Pa air pressure at bounding main level is ~i atm |
| bar (bar, or b) | 1 bar = 100,000 Pa (exactly) ordinarily used in meteorology |
| millibar (mbar, or mb) | grand mbar = 1 bar |
| inches of mercury (in. Hg) | i in. Hg = 3386 Pa used by aviation manufacture, also some weather reports |
| torr | [latex]1 \;\text{torr} = \frac{ane}{760} \;\text{atm}[/latex] named subsequently Evangelista Torricelli, inventor of the barometer |
| millimeters of mercury (mm Hg) | ane mm Hg ~1 torr |
| Table i. Pressure Units | |
Example one
Conversion of Pressure Units
The The states National Atmospheric condition Service reports pressure in both inches of Hg and millibars. Convert a force per unit area of 29.2 in. Hg into:
(a) torr
(b) atm
(c) kPa
(d) mbar
Solution
This is a unit conversion problem. The relationships between the various pressure units are given in Table 1.
(a) [latex]29.2 \;\rule[0.5ex]{2.2em}{0.1ex}\hspace{-2.2em}\text{in Hg} \times \frac{25.iv \;\dominion[0.25ex]{1.2em}{0.1ex}\hspace{-1.2em}\text{mm}}{ane \;\rule[0.25ex]{0.6em}{0.1ex}\hspace{-0.6em}\text{in}} \times \frac{1 \;\text{torr}}{1 \;\rule[0.25ex]{2em}{0.1ex}\hspace{-2em}\text{mm Hg}} = 742 \;\text{torr}[/latex]
(b) [latex]742 \;\rule[0.5ex]{1.8em}{0.1ex}\hspace{-ane.8em}\text{torr} \times \frac{1 \;\text{atm}}{760 \;\rule[0.25ex]{1.2em}{0.1ex}\hspace{-i.2em}\text{torr}} = 0.976 \;\text{atm}[/latex]
(c) [latex]742 \;\dominion[0.5ex]{one.8em}{0.1ex}\hspace{-1.8em}\text{torr} \times \frac{101.325 \;\text{kPa}}{760 \;\rule[0.25ex]{1.0em}{0.1ex}\hspace{-one.0em}\text{torr}} = 98.nine \;\text{kPa}[/latex]
(d) [latex]98.9 \;\rule[0.5ex]{1.9em}{0.1ex}\hspace{-one.9em}\text{kPa} \times \frac{1000 \;\rule[0.25ex]{0.9em}{0.1ex}\hspace{-0.9em}\text{Pa}}{1 \;\rule[0.25ex]{i.1em}{0.1ex}\hspace{-1.1em}\text{kPa}} \times \frac{1 \;\rule[0.25ex]{0.9em}{0.1ex}\hspace{-0.9em}\text{bar}}{100,000 \;\rule[0.25ex]{1.0em}{0.1ex}\hspace{-1.0em}\text{Pa}} \times \frac{1000 \;\text{mbar}}{1 \;\rule[0.25ex]{1.0em}{0.1ex}\hspace{-1.0em}\text{bar}} = 989 \;\text{mbar}[/latex]
Check Your Learning
A typical barometric pressure in Kansas City is 740 torr. What is this pressure in atmospheres, in millimeters of mercury, in kilopascals, and in bar?
Answer:
0.974 atm; 740 mm Hg; 98.7 kPa; 0.987 bar
Nosotros can mensurate atmospheric pressure, the force exerted by the atmosphere on the world'due south surface, with a barometer (Figure 3). A barometer is a glass tube that is closed at one end, filled with a nonvolatile liquid such as mercury, and so inverted and immersed in a container of that liquid. The atmosphere exerts pressure level on the liquid outside the tube, the column of liquid exerts pressure inside the tube, and the pressure at the liquid surface is the same within and outside the tube. The height of the liquid in the tube is therefore proportional to the pressure exerted by the atmosphere.
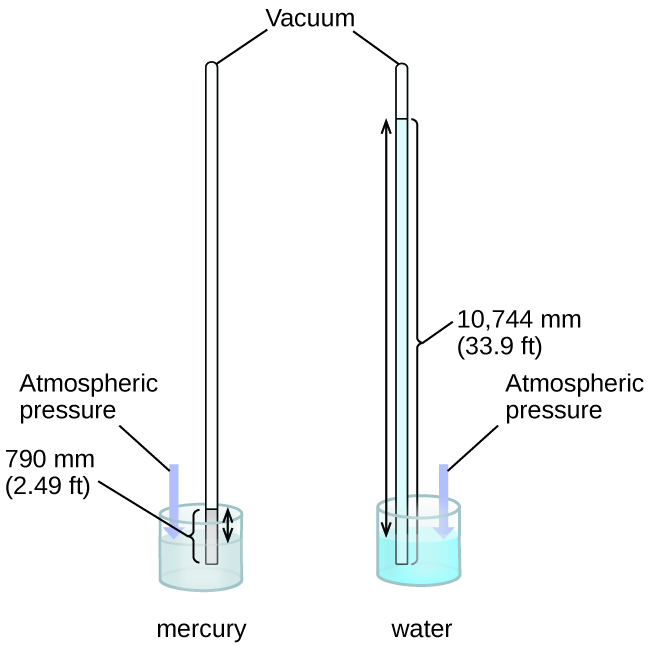
If the liquid is water, normal atmospheric pressure level will back up a cavalcade of h2o over x meters high, which is rather inconvenient for making (and reading) a barometer. Because mercury (Hg) is near 13.6-times denser than water, a mercury barometer merely needs to be [latex]\frac{1}{xiii.6}[/latex] as tall as a water barometer—a more suitable size. Standard atmospheric force per unit area of 1 atm at sea level (101,325 Pa) corresponds to a column of mercury that is about 760 mm (29.92 in.) high. The torr was originally intended to exist a unit equal to i millimeter of mercury, but it no longer corresponds exactly. The pressure exerted by a fluid due to gravity is known as hydrostatic pressure level, p:
[latex]p = h\rho g[/latex]
where h is the acme of the fluid, ρ is the density of the fluid, and chiliad is acceleration due to gravity.
Instance 2
Calculation of Barometric Force per unit area
Show the calculation supporting the claim that atmospheric pressure near body of water level corresponds to the pressure exerted by a cavalcade of mercury that is virtually 760 mm loftier. The density of mercury = 13.half-dozen g/cm3.
Solution
The hydrostatic pressure level is given by p = hρg, with h = 760 mm, ρ = 13.half dozen 1000/cm3, and thou = 9.81 k/due southii. Plugging these values into the equation and doing the necessary unit conversions volition give us the value nosotros seek. (Note: We are expecting to find a pressure level of ~101,325 Pa:)
[latex]101,325 Due north/ \text{grand}^ii = 101,325 \frac{\text{kg} \cdot \text{g/southward}^two}{\text{k}^2} = 101,325 \frac{\text{kg}}{\text{one thousand} \cdot \text{s}^2}[/latex]
[latex]\brainstorm{array}{l @{{}={}}l} p & (760 \;\text{mm} \times \frac{1 \;\text{m}}{g \;\text{mm}}) \times (\frac{13.6 \;\text{yard}}{1 \;\text{cm}^3} \times \frac{i \;\text{kg}}{1000 \;\text{g}} \times \frac{(100 \;\text{cm})^iii}{(1 \;\text{m})^3}) \times (\frac{9.81 \;\text{thou}}{one \;\text{south}^2}) \\[1em] & (0.760 \;\text{m}) (thirteen,600 \;\text{kg/m}^3) (ix.81 \;\text{yard/s}^ii) = 1.01 \times 10^v \;\text{kg/ms}^2 = one.01 \times x^five \;N/ \text{1000}^2 \\[1em] & 1.01 \times 10^v \;\text{Pa} \end{array}[/latex]
Check Your Learning
Calculate the height of a column of water at 25 °C that corresponds to normal atmospheric pressure. The density of h2o at this temperature is 1.0 k/cm3.
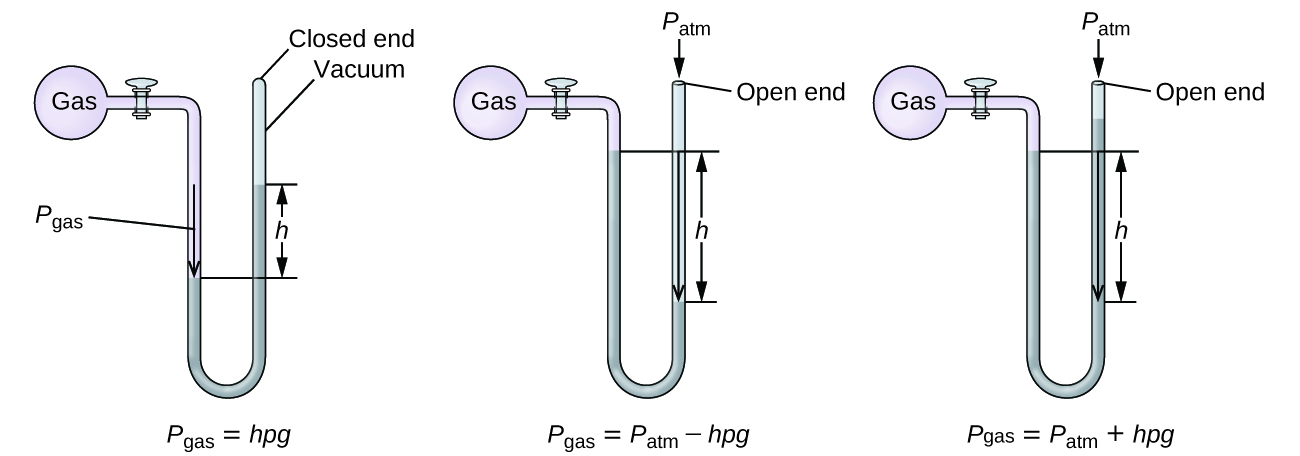
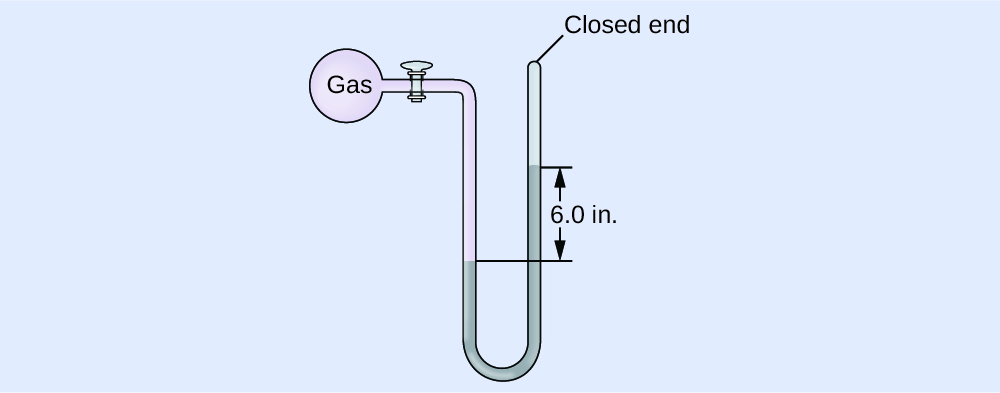
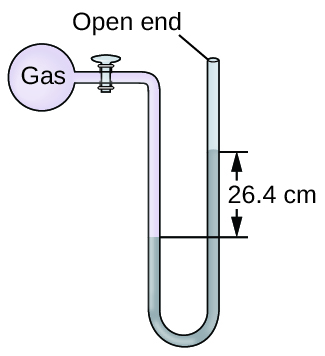
A manometer is a device similar to a barometer that tin be used to mensurate the pressure of a gas trapped in a container. A closed-end manometer is a U-shaped tube with one closed arm, one arm that connects to the gas to be measured, and a nonvolatile liquid (usually mercury) in between. As with a barometer, the distance betwixt the liquid levels in the two arms of the tube (h in the diagram) is proportional to the force per unit area of the gas in the container. An open-finish manometer (Figure iv) is the aforementioned equally a closed-cease manometer, but one of its arms is open to the atmosphere. In this example, the distance between the liquid levels corresponds to the divergence in pressure betwixt the gas in the container and the temper.
Example 3
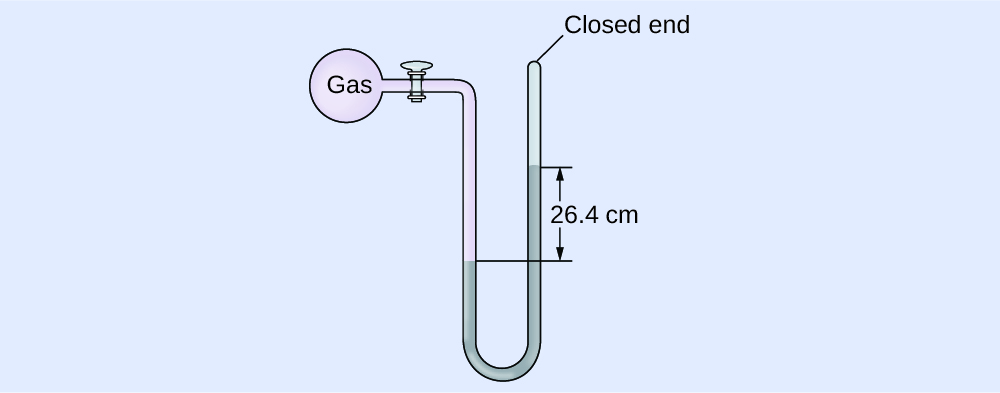
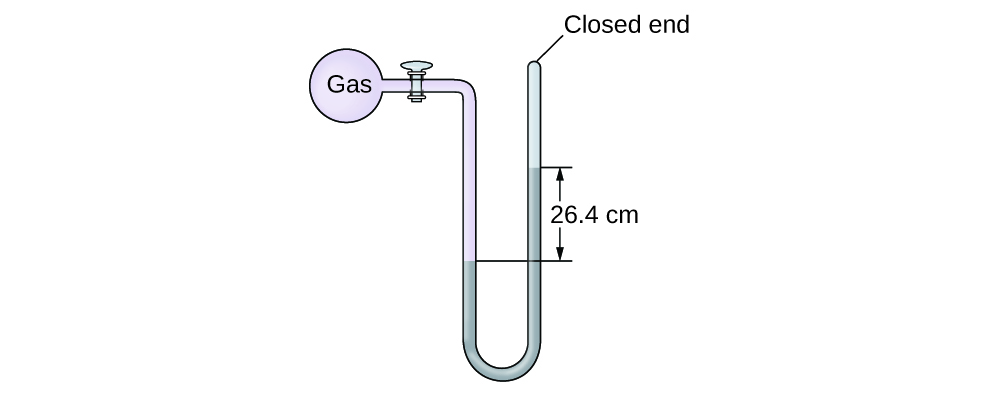
Calculation of Force per unit area Using a Closed-End Manometer
The pressure of a sample of gas is measured with a closed-end manometer, as shown to the correct. The liquid in the manometer is mercury. Make up one's mind the pressure of the gas in:
(a) torr
(b) Pa
(c) bar
Solution
The pressure of the gas is equal to a column of mercury of height 26.four cm. (The pressure at the bottom horizontal line is equal on both sides of the tube. The pressure level on the left is due to the gas and the pressure on the right is due to 26.4 cm Hg, or mercury.) Nosotros could use the equation p = hρg every bit in Example ii, merely it is simpler to merely convert betwixt units using Table ane.
(a) [latex]26.4 \;\rule[0.5ex]{two.8em}{0.1ex}\hspace{-2.8em}\text{cm Hg} \times \frac{ten \;\dominion[0.25ex]{ii.5em}{0.1ex}\hspace{-2.5em}\text{mm Hg}}{one \;\rule[0.25ex]{two.5em}{0.1ex}\hspace{-2.5em}\text{mm Hg}} \times \frac{one \;\text{torr}}{1 \;\rule[0.25ex]{ii.5em}{0.1ex}\hspace{-2.5em}\text{mm Hg}} = 264 \;\text{torr}[/latex]
(b) [latex]264 \;\dominion[0.5ex]{ane.7em}{0.1ex}\hspace{-ane.7em}\text{torr} \times \frac{ane \;\rule[0.25ex]{1.3em}{0.1ex}\hspace{-one.3em}\text{atm}}{760 \;\rule[0.25ex]{ane.3em}{0.1ex}\hspace{-1.3em}\text{torr}} \times \frac{101,325 \;\text{Pa}}{1 \;\dominion[0.25ex]{1.3em}{0.1ex}\hspace{-ane,3em}\text{atm}} = 35,200 \;\text{Pa}[/latex]
(c) [latex]35,200 \;\rule[0.5ex]{1.2em}{0.1ex}\hspace{-1.2em}\text{Pa} \times \frac{1 \;\text{bar}}{100,000 \;\dominion[0.25ex]{1em}{0.1ex}\hspace{-1em}\text{Pa}} = 0.352 \;\text{bar}[/latex]
Bank check Your Learning
The pressure of a sample of gas is measured with a closed-end manometer. The liquid in the manometer is mercury. Determine the pressure of the gas in:
(a) torr
(b) Pa
(c) bar
Answer:
(a) ~150 torr; (b) ~20,000 Pa; (c) ~0.twenty bar
Example 4
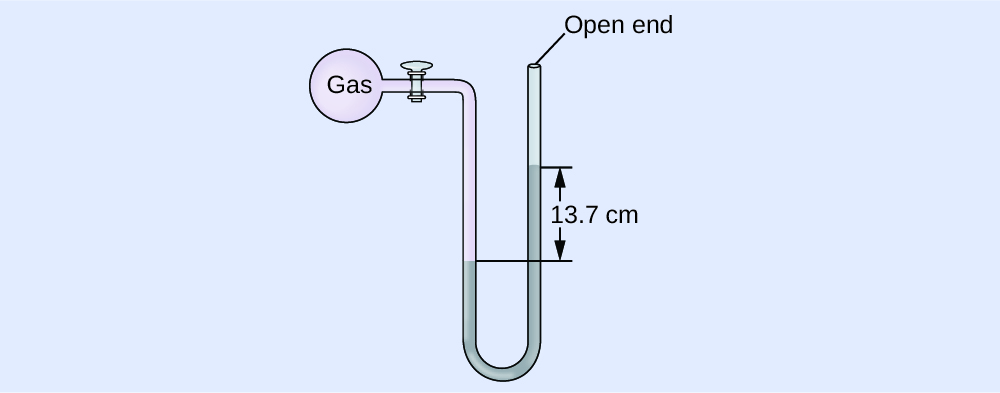
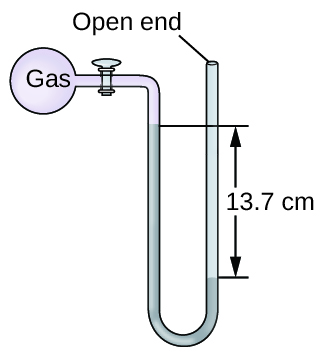
Calculation of Force per unit area Using an Open up-End Manometer
The pressure of a sample of gas is measured at sea level with an open up-finish Hg (mercury) manometer, every bit shown to the right. Determine the pressure of the gas in:
(a) mm Hg
(b) atm
(c) kPa
Solution
The pressure of the gas equals the hydrostatic pressure due to a column of mercury of height 13.7 cm plus the pressure of the atmosphere at sea level. (The force per unit area at the bottom horizontal line is equal on both sides of the tube. The pressure on the left is due to the gas and the pressure on the right is due to thirteen.7 cm of Hg plus atmospheric pressure.)
(a) In mm Hg, this is: 137 mm Hg + 760 mm Hg = 897 mm Hg
(b) [latex]897 \;\dominion[0.5ex]{3em}{0.1ex}\hspace{-3em}\text{mm Hg} \times \frac{1 \;\text{atm}}{760 \;\rule[0.25ex]{2.5em}{0.1ex}\hspace{-two.5em}\text{mm Hg}} = 1.18 \;\text{atm}[/latex]
(c) [latex]1.eighteen \;\rule[0.5ex]{i.8em}{0.1ex}\hspace{-1.8em}\text{atm} \times \frac{101.325 \;\text{kPa}}{1 \;\rule[0.25ex]{one.5em}{0.1ex}\hspace{-one.5em}\text{atm}} = i.twenty \times 10^2 \;\text{kPa}[/latex]
Check Your Learning
The force per unit area of a sample of gas is measured at sea level with an open-cease Hg manometer, as shown to the right. Make up one's mind the pressure of the gas in:
(a) mm Hg
(b) atm
(c) kPa
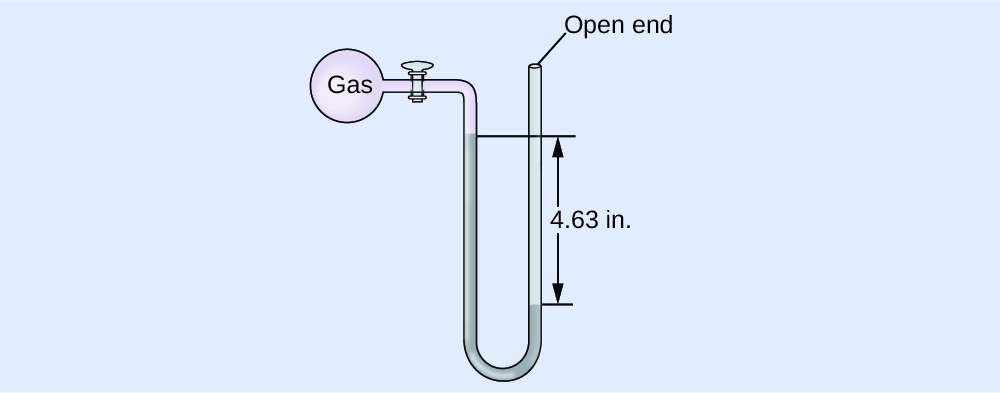
Answer:
(a) 642 mm Hg; (b) 0.845 atm; (c) 85.vi kPa
Measuring Blood Pressure level
Claret pressure is measured using a device called a sphygmomanometer (Greek sphygmos = "pulse"). It consists of an inflatable cuff to restrict blood flow, a manometer to measure the pressure level, and a method of determining when blood flow begins and when it becomes impeded (Effigy 5). Since its invention in 1881, information technology has been an essential medical device. There are many types of sphygmomanometers: manual ones that require a stethoscope and are used by medical professionals; mercury ones, used when the about accuracy is required; less accurate mechanical ones; and digital ones that tin can be used with fiddling grooming but that take limitations. When using a sphygmomanometer, the gage is placed around the upper arm and inflated until blood flow is completely blocked, then slowly released. As the eye beats, blood forced through the arteries causes a ascension in pressure. This rise in pressure at which blood flow begins is the systolic pressure—the peak force per unit area in the cardiac bicycle. When the cuff's pressure equals the arterial systolic pressure, blood flows past the cuff, creating aural sounds that tin be heard using a stethoscope. This is followed past a decrease in pressure as the middle's ventricles prepare for another beat out. As cuff pressure level continues to decrease, eventually audio is no longer heard; this is the diastolic pressure—the lowest pressure (resting stage) in the cardiac bicycle. Blood pressure units from a sphygmomanometer are in terms of millimeters of mercury (mm Hg).

Meteorology, Climatology, and Atmospheric Science
Throughout the ages, people have observed clouds, winds, and precipitation, trying to discern patterns and make predictions: when information technology is best to plant and harvest; whether it is safe to set out on a sea voyage; and much more. We now face complex weather and atmosphere-related challenges that volition have a major bear upon on our civilization and the ecosystem. Several different scientific disciplines use chemical principles to assist us better sympathise weather, the atmosphere, and climate. These are meteorology, climatology, and atmospheric science. Meteorology is the study of the atmosphere, atmospheric phenomena, and atmospheric effects on globe's weather. Meteorologists seek to understand and predict the atmospheric condition in the short term, which can save lives and benefit the economy. Weather forecasts (Figure half-dozen) are the result of thousands of measurements of air pressure, temperature, and the like, which are compiled, modeled, and analyzed in weather centers worldwide.
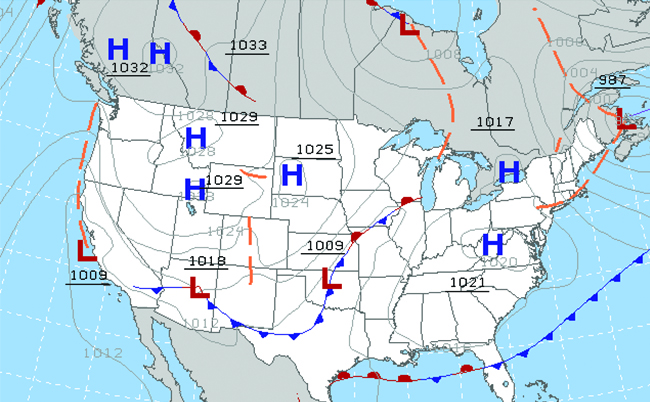
In terms of weather, depression-pressure level systems occur when the earth's surface atmospheric pressure is lower than the surrounding environment: Moist air rises and condenses, producing clouds. Movement of wet and air within various atmospheric condition fronts instigates most weather events.
The temper is the gaseous layer that surrounds a planet. Earth'south atmosphere, which is roughly 100–125 km thick, consists of roughly 78.one% nitrogen and 21.0% oxygen, and can exist subdivided further into the regions shown in Effigy 7: the exosphere (furthest from globe, > 700 km above sea level), the thermosphere (80–700 km), the mesosphere (50–80 km), the stratosphere (second lowest level of our atmosphere, 12–50 km higher up body of water level), and the troposphere (up to 12 km to a higher place body of water level, roughly eighty% of the earth's atmosphere by mass and the layer where most weather events originate). As you go higher in the troposphere, air density and temperature both decrease.
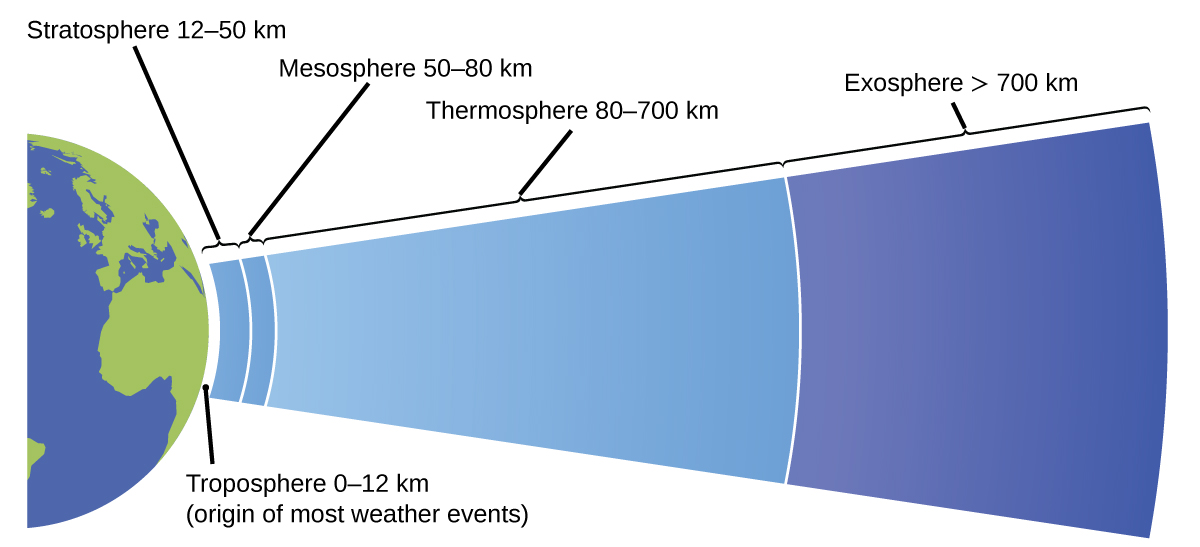
Climatology is the written report of the climate, averaged weather atmospheric condition over long time periods, using atmospheric information. However, climatologists written report patterns and furnishings that occur over decades, centuries, and millennia, rather than shorter fourth dimension frames of hours, days, and weeks like meteorologists. Atmospheric science is an even broader field, combining meteorology, climatology, and other scientific disciplines that study the atmosphere.
Key Concepts and Summary
Gases exert pressure, which is pressure level. The pressure level of a gas may be expressed in the SI unit of pascal or kilopascal, too as in many other units including torr, temper, and bar. Atmospheric pressure level is measured using a barometer; other gas pressures can be measured using i of several types of manometers.
Key Equations
- [latex]P = \frac{F}{A}[/latex]
- [latex]p = h \rho 1000[/latex]
Chemical science End of Chapter Exercises
- Why are abrupt knives more effective than dull knives (Hint: think virtually the definition of pressure level)?
- Why exercise some small bridges take weight limits that depend on how many wheels or axles the crossing vehicle has?
- Why should you coil or belly-crawl rather than walk across a thinly-frozen pond?
- A typical barometric force per unit area in Redding, California, is about 750 mm Hg. Summate this force per unit area in atm and kPa.
- A typical barometric pressure in Denver, Colorado, is 615 mm Hg. What is this pressure in atmospheres and kilopascals?
- A typical barometric pressure in Kansas Metropolis is 740 torr. What is this force per unit area in atmospheres, in millimeters of mercury, and in kilopascals?
- Canadian tire pressure gauges are marked in units of kilopascals. What reading on such a judge corresponds to 32 psi?
- During the Viking landings on Mars, the atmospheric pressure was determined to be on the average about vi.50 millibars (one bar = 0.987 atm). What is that pressure in torr and kPa?
- The pressure of the atmosphere on the surface of the planet Venus is about 88.8 atm. Compare that pressure in psi to the normal pressure on earth at sea level in psi.
- A medical laboratory catalog describes the pressure in a cylinder of a gas every bit fourteen.82 MPa. What is the pressure of this gas in atmospheres and torr?
- Consider this scenario and answer the following questions: On a mid-August solar day in the northeastern United States, the following information appeared in the local newspaper: atmospheric pressure at sea level 29.97 in., 1013.9 mbar.
(a) What was the pressure level in kPa?
(b) The pressure nearly the seacoast in the northeastern Us is usually reported near xxx.0 in. Hg. During a hurricane, the pressure may fall to nearly 28.0 in. Hg. Calculate the drop in pressure in torr.
- Why is information technology necessary to use a nonvolatile liquid in a barometer or manometer?
- The force per unit area of a sample of gas is measured at sea level with a closed-end manometer. The liquid in the manometer is mercury. Determine the pressure level of the gas in:
(a) torr
(b) Pa
(c) bar
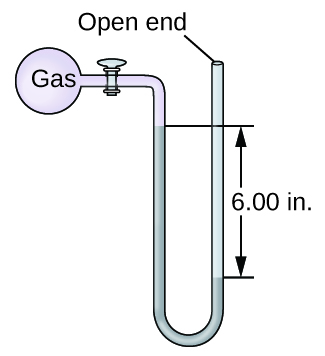
- The pressure of a sample of gas is measured with an open-terminate manometer, partially shown to the right. The liquid in the manometer is mercury. Bold atmospheric pressure level is 29.92 in. Hg, determine the force per unit area of the gas in:
(a) torr
(b) Pa
(c) bar
- The pressure of a sample of gas is measured at sea level with an open-end mercury manometer. Assuming atmospheric pressure is 760.0 mm Hg, decide the pressure of the gas in:
(a) mm Hg
(b) atm
(c) kPa
- The pressure of a sample of gas is measured at bounding main level with an open-terminate mercury manometer. Assuming atmospheric pressure is 760 mm Hg, determine the pressure of the gas in:
(a) mm Hg
(b) atm
(c) kPa
- How would the use of a volatile liquid affect the measurement of a gas using open up-ended manometers vs. closed-end manometers?
Glossary
- temper (atm)
- unit of measurement of pressure; 1 atm = 101,325 Pa
- bar
- (bar or b) unit of pressure; 1 bar = 100,000 Pa
- barometer
- device used to measure atmospheric pressure
- hydrostatic pressure
- pressure exerted past a fluid due to gravity
- manometer
- device used to measure the pressure level of a gas trapped in a container
- pascal (Pa)
- SI unit of pressure level; ane Pa = 1 N/m2
- pounds per square inch (psi)
- unit of measurement of pressure common in the United states
- pressure
- forcefulness exerted per unit surface area
- torr
- unit of pressure; [latex]i \;\text{torr} = \frac{1}{760} \;\text{atm}[/latex]
Solutions
Answers to Chemistry End of Chapter Exercises
one. The cutting edge of a knife that has been sharpened has a smaller surface area than a dull knife. Since pressure is force per unit surface area, a sharp knife will exert a higher pressure with the same amount of force and cut through textile more effectively.
3. Lying down distributes your weight over a larger surface area, exerting less pressure on the water ice compared to standing upwardly. If you exert less pressure, you are less likely to break through thin ice.
v. 0.809 atm; 82.0 kPa
7. 2.2 × xtwo kPa
ix. Earth: fourteen.vii lb in–two; Venus: 13.1 × x3 lb in−2
11. (a) 101.5 kPa; (b) 51 torr drop
13. (a) 264 torr; (b) 35,200 Pa; (c) 0.352 bar
xv. (a) 623 mm Hg; (b) 0.820 atm; (c) 83.1 kPa
17. With a closed-end manometer, no alter would be observed, since the vaporized liquid would contribute equal, opposing pressures in both artillery of the manometer tube. However, with an open-ended manometer, a higher pressure reading of the gas would be obtained than expected, since P gas = P atm + P vol liquid.
What Size Tank Would Be Needed To Contain This Same Amount Of Helium At Atmospheric Pressure (1 Atm),
Source: https://opentextbc.ca/chemistry/chapter/9-1-gas-pressure/
Posted by: pattondesten.blogspot.com


0 Response to "What Size Tank Would Be Needed To Contain This Same Amount Of Helium At Atmospheric Pressure (1 Atm)"
Post a Comment